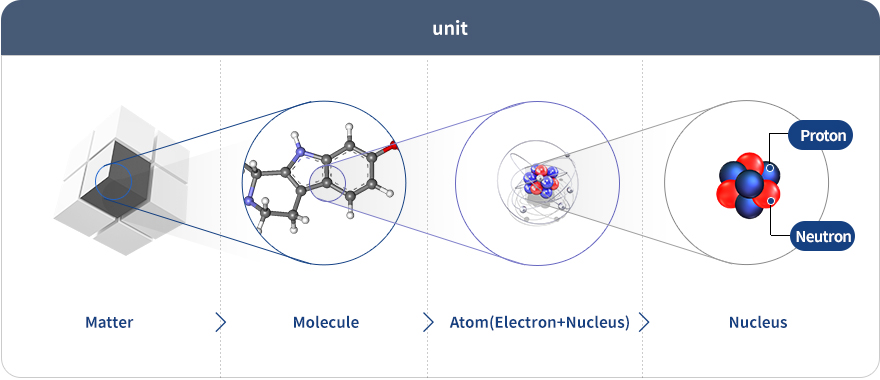
Atoms are basic units of nature. Atoms are made of protons, neutrons and electrons. Atomic properties are determined by the number of such constituents. The number of protons determines the atomic number and chemical properties, while the atomic mass is equal to the sum of protons and neutrons.
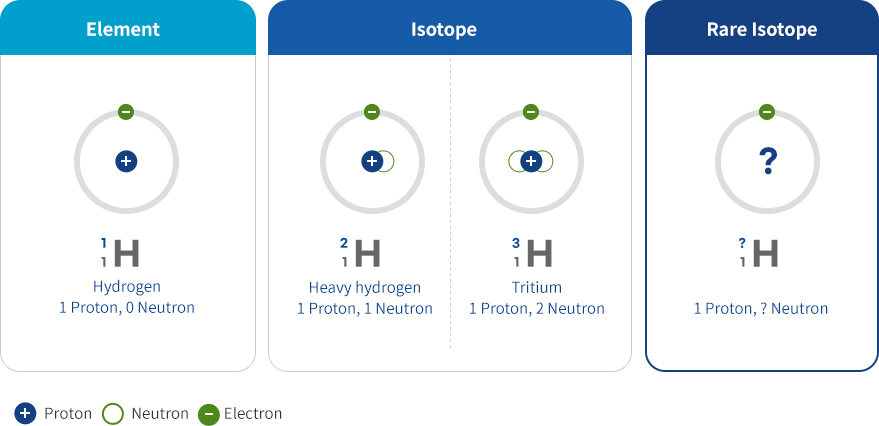
Isotopes are twin atoms with the same number of protons and thus the same atomic number but different numbers of neutrons. Each element has several different isotopes. Scientists have discovered 118 elements and about 3,000 isotopes to date.
Rare isotopes are isotopes that have not yet been discovered because they are rare and decay rapidly. They must be produced or discovered by artificial means because they no longer exist or only remain in very small traces on the earth.
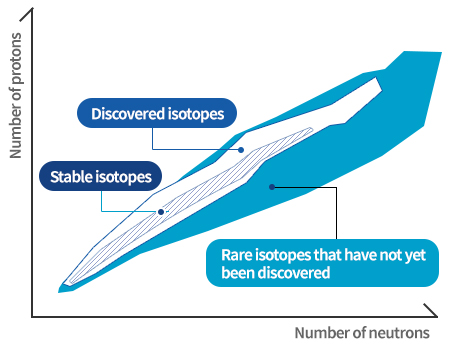
The nuclear chart shows the distribution of stable isotopes existing on the earth, artificially discovered isotopes, and rare isotopes that have not yet been discovered. Scientists believe that there are a greater number of undiscovered rare isotopes than the number of known atoms.
The discovery of new phenomena is valuable in itself, and creates new knowledge for humanity. Rare isotopes can be utilized to obtain new knowledge that was not accessible with existing techniques. In particular, rare isotopes enable better understanding of the creation and evolution of the natural world and the universe, and the characteristics of substances. This implies that rare isotopes have the potential to break free of the limits of current basic science and create new research fields.
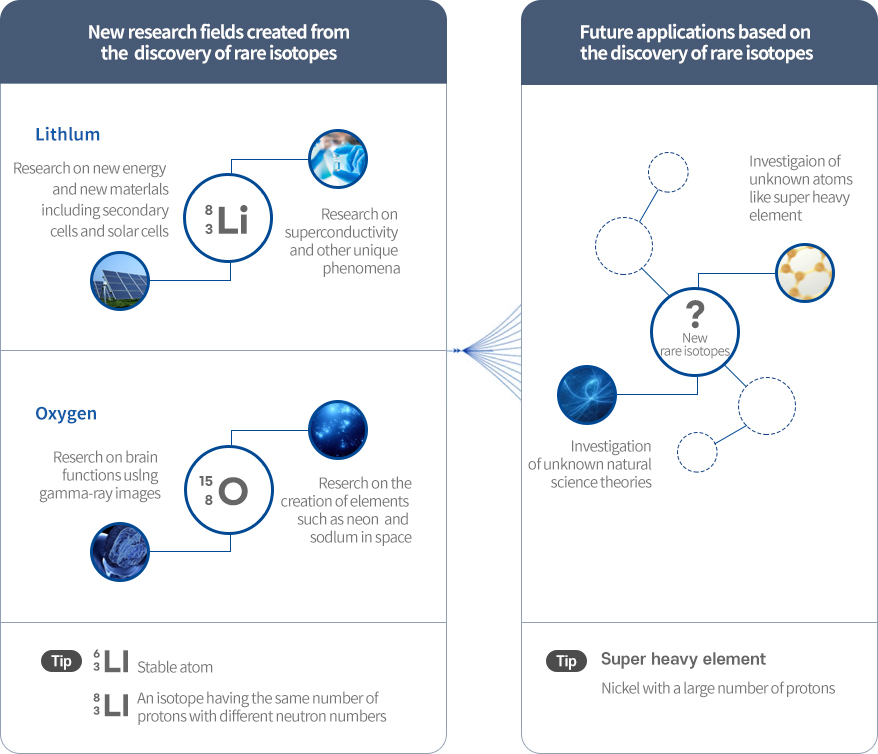
New rare isotopes - Investigation of unknown natural science theories, Investigaion of unknown atoms like super heavy element
Tip : Super heavy element -Nickel with a large number of protons